Products
What are the applications of AP-CAD software?
Why is AP-CAD software needed?
When can AP-CAD software be applied?
How is AP-CAD software implemented?
AP-CAD stands for Advanced Pharmaceutics – Computational Analysis and Design. This software now contains five application modules
- Drug release kinetics prediction – analytical solution
- Drug release kinetics prediction – coating system
- Drug release kinetics prediction – matrix system
- Dosage form parameter estimation
- Pharmaceutical material property estimation
The first module includes most of currently available exact or approximate analytical solutions for predicting drug release kinetics of various dosage forms. They are grouped according to release mechanisms, geometries, initial drug loadings and boundary conditions. A typical window is shown as below:

In the second module, drug release from various coated dosage forms can be simulated. The state of initial drug loading can be dispersed, dissolved, or both and its values can be above or below drug solubility. Release mechanisms such as dissolution controlled, diffusion controlled or dissolution-diffusion controlled are considered. The coating thickness can be directly inputted if the value is known or be calculated by providing basic information such as weight gain and initial batch weight. There are no analytical solutions available to describe a complete release process of a coated dosage form, thus numerical solutions such as finite element and finite difference methods are used in the computational procedure. A typical window is shown as below:

In the third module, drug release from various polymeric matrix dosage forms can be simulated. The state of initial drug loading can be dispersed, dissolved, or both and its values can be above or below drug solubility. Release mechanisms such as dissolution controlled, diffusion controlled or dissolution-diffusion controlled are considered. There are no analytical solutions available to describe a complete release process of a complex matrix dosage form, thus numerical solutions such as finite element and finite difference methods are used in the computational procedure. A typical window is shown as below:
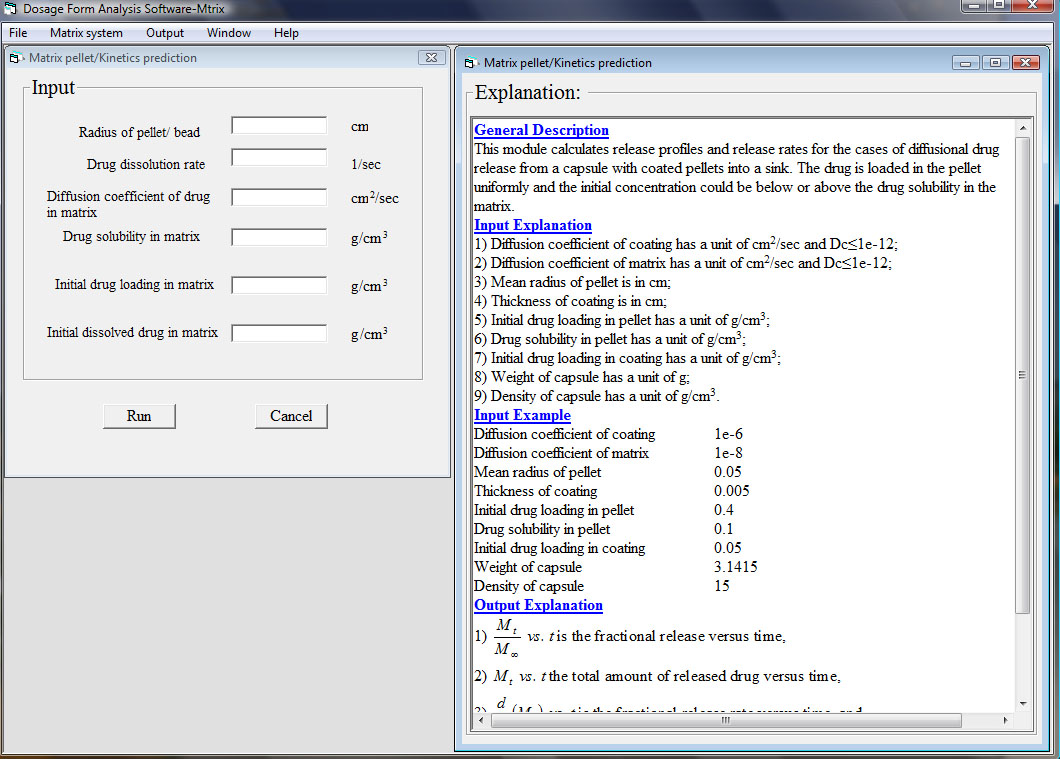
The fourth module is used to identify design parameters or formulation variables, for example dimensions, material properties, or initial drug loadings, based on experimental data by using a suitable release mechanism model. A typical window is shown as below:
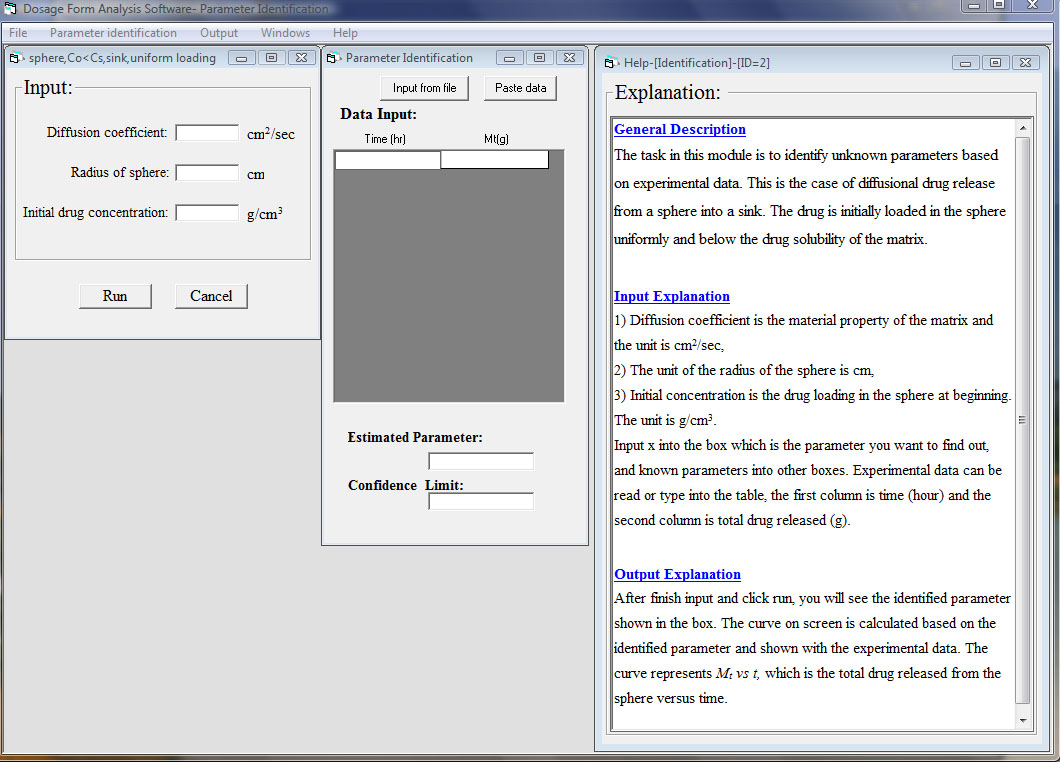
In the fifth module, material parameters such as diffusion coefficients, drug solubilities, and partition coefficients can be estimated based on structural related models. Basic chemical compound information is used as inputs or it might be calculated by the software if unknown such as molar volume of solute. A typical window is shown as below:
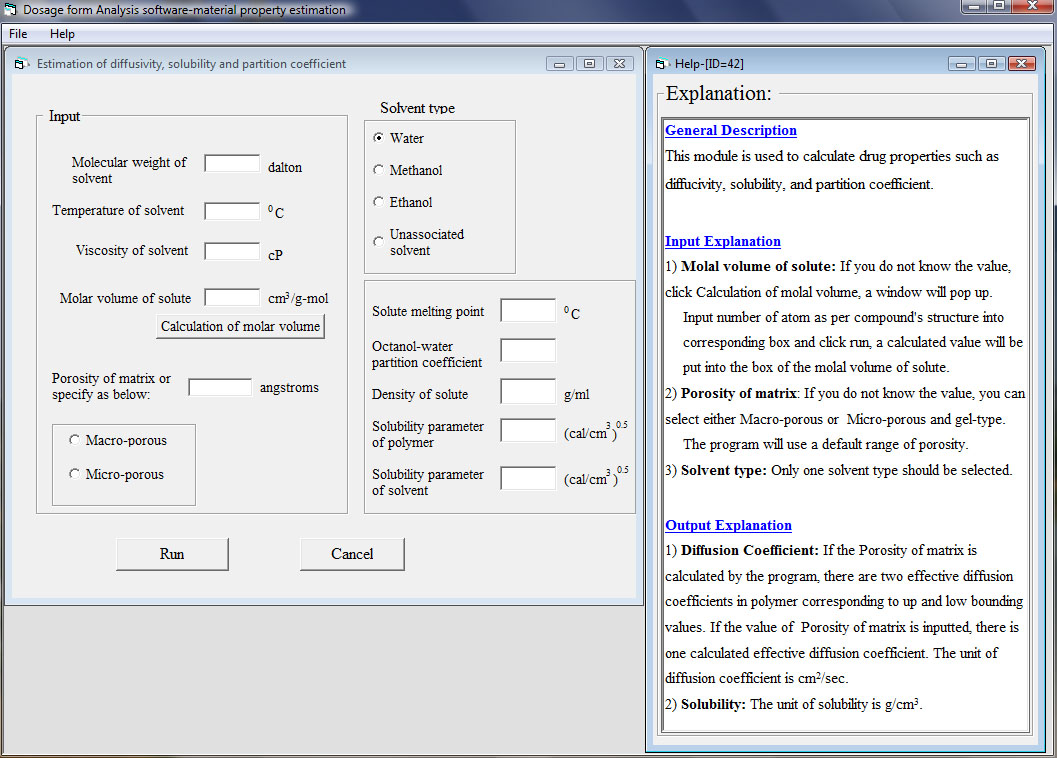
Why is AP-CAD software needed?
Because of the complexity, risk, and cost of dosage form design and development, pharmaceutical scientists need to apply the best scientific methods and technology in their daily work. AP-CAD provides useful insight at every stage of the process to reduce amount of prototype testing, to allow multiple “what-if” scenarios to be checked quickly and effectively, t o simulate designs that are difficult and/or too costly to test . The bottom line is cost saving, time saving, and create more reliable, better-quality products. By using this tool scientists have more time and freedom to analyze, draw conclusions, capitalize on R&D knowledge and meet the requirements of Quality by Design proposed by FDA.
When can AP-CAD software be applied?
Essentially AP-CAD can be applied at every stage of dosage form development process.
Early stage of dosage form development
- Examine formulation/design options
- Test design concepts
Middle stage of dosage form development
- Estimate material and kinetic parameters
- Investigate influences of design factors
- Optimize formulation and device
- Verify various “what-if” scenarios
Late stage of dosage form development
- Predict in vivo performance of the dosage form
- Establish in vitro-in vivo correlation
How is AP-CAD software implemented?
Using the software to analyze a dosage form, four steps need to be followed:
- Understand the release mechanism and the delivery system
- Select a suitable model and determine model parameters
- Interpret results and realize discrepancies
- Optimize the design to achieve objectives
AP-CAD has a very user-friendly interface and most of the inputs are self-explaining. An explanation window is always accompanied with the input and output windows. Detailed description for each input and output are given and an input sample is provided to help the user. An example of analysis of a two dimensional coated tablet is given as follows:
In the AP-CAD main directory, click on AP-CAD and a main menu will pop up.

Select “release kinetics prediction coating system”. The coating system window will appear:
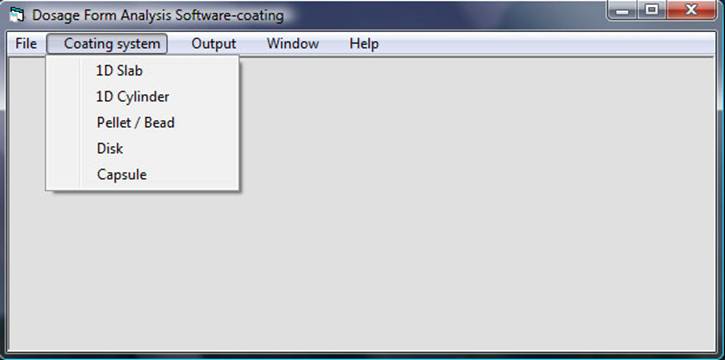
Click on “Coating System” in the top menu bar and select “disk” in the drop-down menu. An input window will appear:
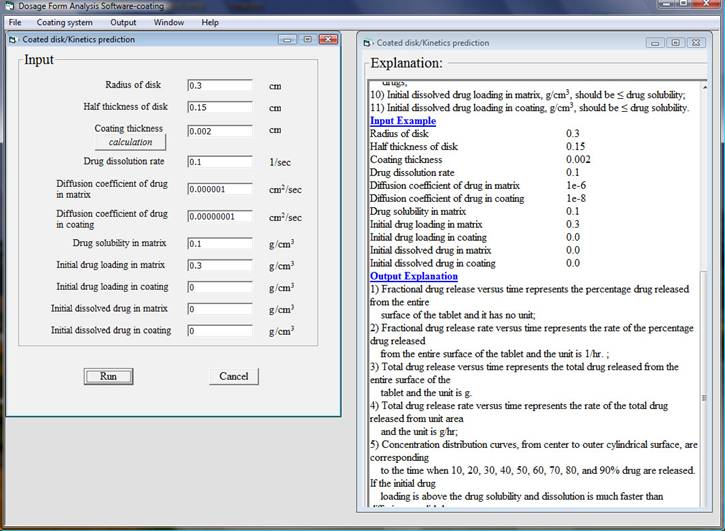
After finishing inputting data and click run, for a computer with 2.4 GHz CPU speed and 3 GB random memory it takes less than two minutes to do the analysis then an output window will appear. There are five output results and simply chose one of them, the corresponding plot will be shown. In this example total drug release versus time, total drug release rate versus time, and concentration distribution and moving boundary are presented as follows.
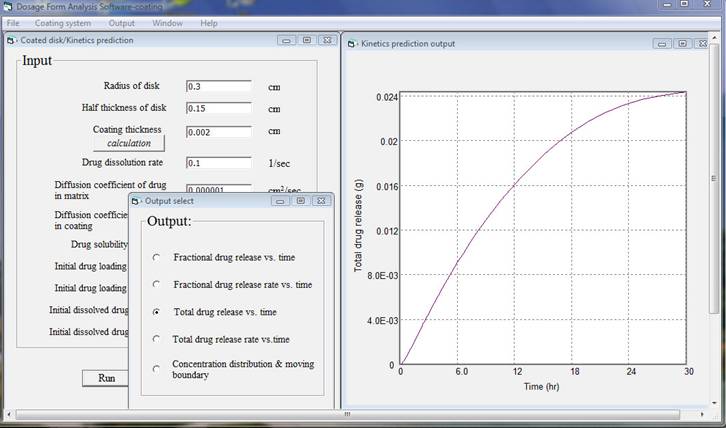
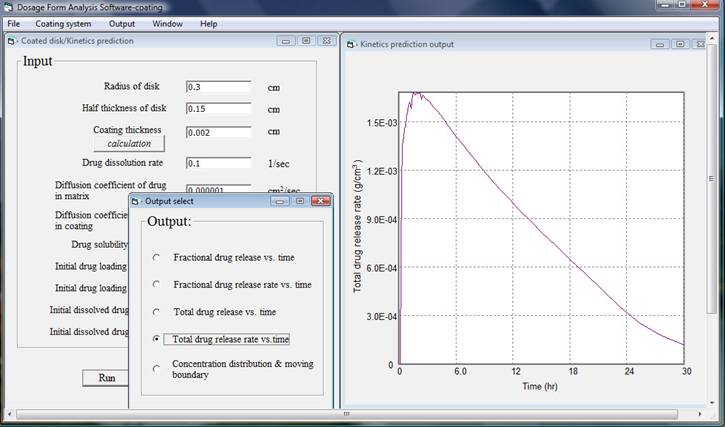
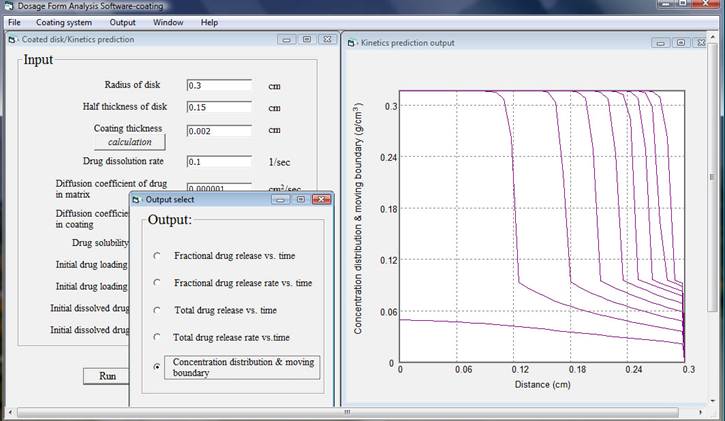
By changing input data, formulation scientists will be able to reveal the effects of formulation variables on the release kinetics and asses the relative differences due to various geometries, dimensions, material properties, initial drug loading, and environments. It is also very important to keep in mind that the assumptions used in the analysis, the limitation of the simulation model, and the possible discrepancies of the input data.